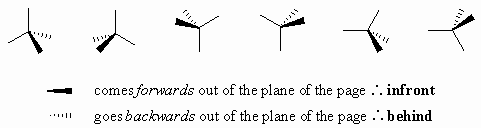
Wedge-dash diagrams
Usually drawn with two bonds in the plane of the page, one infront, and one behind to give the molecule perspective. When drawing wedge-dash it is a good idea to visualise the tetrahedral arrangement of the groups and try to make the diagram "fit" this. As a suggestion, they seem to be most effective when the "similar" pairs of bonds (2-in-plane, 2-out-of-plane) are next to each other, see below:
Sawhorse
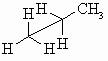 Sawhorse diagrams are similar to wedge-dash diagrams, but without
trying to use "shading" to denote the perspective. The representation to the
right of propane has been drawn so that we are looking at the molecule which
is below us and to our left.
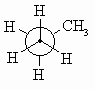
Sawhorse diagrams are similar to wedge-dash diagrams, but without
trying to use "shading" to denote the perspective. The representation to the
right of propane has been drawn so that we are looking at the molecule which
is below us and to our left.
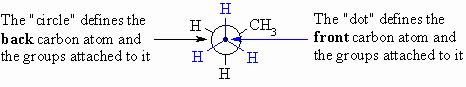
Newman Projections
 These
projections are drawn by looking directly along a particular bond in the system
(here a C-C bond) and arranging the substituents symmetrically around the atoms
at each end of that bond. The protocol requires that the atoms within the central
bond are defined as shown below:
These
projections are drawn by looking directly along a particular bond in the system
(here a C-C bond) and arranging the substituents symmetrically around the atoms
at each end of that bond. The protocol requires that the atoms within the central
bond are defined as shown below:
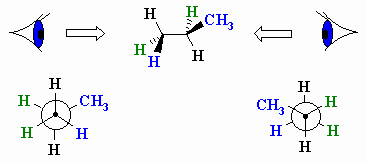
In order to draw a Newman projection from a wedge-dash diagram,
it is useful to imagine putting your "eye" in line with the central bond in
order to look along it.
Let's work through an example, consider drawing a Newman projection by looking at the following wedge-dash diagram of propane from the left hand side.
Drawing Cyclohexanes
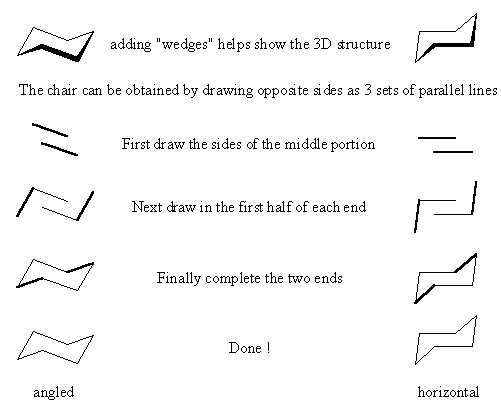
Drawing cyclohexane so that it looks like a chair can be the key to appreciating the axial and equatorial positions. If you are unable to draw good looking structures that clearly show axial and equatorial positions, then your instructor is probably going to assume that you don't know.
By not mastering the trick of drawing cyclohexanes the only person that really suffers is you the student. You deprive yourself of the knowledge and the chance to appreciate it and what it means. Believe me, it will be needed later.
The first step is drawing the chair itself. Although the chair "looks better" when slightly angled, it maybe easier to "learn" to draw it with the middle portion horizontal.
 Cycloalkanes
Cycloalkanes
Usually drawn with two bonds in the plane of the page, one infront, and one behind to give the molecule perspective. When drawing wedge-dash it is a good idea to visualise the tetrahedral arrangement of the groups and try to make the diagram "fit" this. As a suggestion, they seem to be most effective when the "similar" pairs of bonds (2-in-plane, 2-out-of-plane) are next to each other, see below:

 Sawhorse diagrams are similar to wedge-dash diagrams, but without
trying to use "shading" to denote the perspective. The representation to the
right of propane has been drawn so that we are looking at the molecule which
is below us and to our left.
Sawhorse diagrams are similar to wedge-dash diagrams, but without
trying to use "shading" to denote the perspective. The representation to the
right of propane has been drawn so that we are looking at the molecule which
is below us and to our left. Newman Projections
 These
projections are drawn by looking directly along a particular bond in the system
(here a C-C bond) and arranging the substituents symmetrically around the atoms
at each end of that bond. The protocol requires that the atoms within the central
bond are defined as shown below:
These
projections are drawn by looking directly along a particular bond in the system
(here a C-C bond) and arranging the substituents symmetrically around the atoms
at each end of that bond. The protocol requires that the atoms within the central
bond are defined as shown below: 
Let's work through an example, consider drawing a Newman projection by looking at the following wedge-dash diagram of propane from the left hand side.
- First draw the dot and circle to represent the front and back C respectively
- Since the front carbon atom has an H atom in the plane of the page pointing up we can add that first
- The back carbon atom has an H atom in the plane of the page pointing down
- Now add the other bonds to each C so that it is symmetrical
- The groups / bonds (blue) that were forward of the plane of the page in the original wedge-dash diagram are now to our right
- Those behind (green) the plane are now to our left

- Now you try the same thing, but looking from the right to generate the other Newman projection.
Drawing Cyclohexanes
Drawing cyclohexane so that it looks like a chair can be the key to appreciating the axial and equatorial positions. If you are unable to draw good looking structures that clearly show axial and equatorial positions, then your instructor is probably going to assume that you don't know.
By not mastering the trick of drawing cyclohexanes the only person that really suffers is you the student. You deprive yourself of the knowledge and the chance to appreciate it and what it means. Believe me, it will be needed later.
The first step is drawing the chair itself. Although the chair "looks better" when slightly angled, it maybe easier to "learn" to draw it with the middle portion horizontal.
 Cycloalkanes
Cycloalkanes - Cycloalkanes just means "cyclic alkanes" - ring systems formed of only C-C and C-H bonds.
- Such structures are commonly encountered in natural compounds such as steroids, with cyclopentanes and cyclohexanes being the most common.
- Other than cyclopropane (which must be planar), cycloalkanes are "puckered" to relieve some of the ring strain by lowering angle and torsional strains.
- Ring strain : cyclopropane > cyclobutane > cyclopentane > cyclohexane
| C3H6 CYCLOPROPANE ΔHc / C7 kJ/mol (-166.6 kcal/mol) |
||
| C4H8 CYCLOBUTANE ΔHc / CH2 = -681 kJ/mol (-162.7 kcal/mol) |
C5H10  CYCLOPENTANE ΔHc / CH2 = -658 kJ/mol (-157.3 kcal/mol) |
||
No comments:
Post a Comment